Background and Significance:
Poor stem cell mobilization with failure to produce the target cell dose of ≥2x10 6 CD34 cells/kg required for autologous stem cell transplant (ASCT) occurs in 15-50% of patients with lymphoma or multiple myeloma (MM). Newer therapies may also have a negative impact on mobilization, supporting a need for newer treatment options. Burixafor (GPC-100) is a potent and selective small molecule antagonist of CXCR4. Previous clinical trials with burixafor alone or in combination with G-CSF have demonstrated safe and effective mobilization of stem cells. Preclinical murine studies demonstrated that burixafor in combination with propranolol is a potent mobilizer of white blood cells and mouse stem cells, and mobilization was further augmented with the addition of G-CSF. The goal of the current clinical study is to evaluate the efficacy of burixafor and propranolol in the presence and absence of G-CSF. The triple combination is predicted to be best in class and can target patients at risk for poor mobilization while burixafor plus propranolol will allow for an option when G-CSF is not tolerated, such as in sickle cell disease or patients with autoimmune disease.
Study Design and Methods:
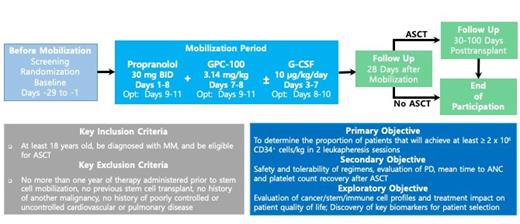
This study (NCT05561751) is an open-label, randomized, multi-site (U.S. only) Phase 2 trial. Approximately 40 participants will be enrolled across 8-10 sites and randomized in a 1:1 ratio to one of two treatment arms: Burixafor in combination with propranolol or burixafor in combination with propranolol and G-CSF. The study will employ a Bayesian Optimal Phase 2 (BOP2) design for participant enrollment to characterize the safety and clinical activity of burixafor. Participants must be at least 18 years of age, be diagnosed with MM and be eligible for ASCT. Key exclusion criteria include: no more than one year of therapy administered prior to stem cell mobilization, previous stem cell transplant, history of another malignancy, and history of poorly controlled or uncontrolled cardiovascular or pulmonary disease.
All participants will self-administer 30 mg propranolol orally twice daily from Days 1 to 8. For participants in the treatment arm receiving G-CSF, they will receive SC injections of 10g/kg/day G-CSF in the afternoon on Days 3 to 7. On days 7 and 8, participants will receive 3.14 mg/kg dose of burixafor via IV and undergo leukapheresis for stem cell collection two hours post drug administration. Participants may undergo additional optional days of the treatment regimen and stem cell collection, per Investigator's discretion to meet institutional standards.
The primary objective is to determine the proportion of patients that will achieve at least ≥2 x 10 6 CD34 + cells/kg in 2 leukapheresis sessions following the treatment regimens. Key secondary objectives include: assessment of safety and tolerability of GPC-100 and propranolol with and without G-CSF, evaluation of the pharmacodynamics by determining levels of circulating CD34 + cell counts and levels of the chemokine CXCL12 in peripheral blood, and assessment of mean time to ANC and platelet count recovery after ASCT. Exploratory objectives are also included to discover key biomarkers for patient selection, to evaluate cancer/stem/immune cell profiles, and to evaluate treatment impact on patient quality of life.
OffLabel Disclosure:
Caculitan:GPCR Therapeutics, Inc.: Current Employment, Current holder of stock options in a privately-held company. Cardarelli:GPCR Therapeutics: Current Employment, Current holder of stock options in a privately-held company. Khouri:Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events; GPCR Therapeutics: Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events. Suh:Kite Pharma: Membership on an entity's Board of Directors or advisory committees. Ramanathan:spouse owns stock options in Smith & Nephew: Current equity holder in publicly-traded company; spouse owns stocks in Alcon, biogen, boston scientific, dexcom, gilead, intuitive surgical, Moderna, Medtronic, Novartis,tandem diabetes, ocular therapeutics,Pfizer,Sanofi,vertex, viatris,: Current equity holder in publicly-traded company; spouse divested stocks in abbvie, Jansenn and BMS: Divested equity in a private or publicly-traded company in the past 24 months.
Propranolol is used to treat high blood pressure, performance anxiety, etc.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal